 |
ROCKET THERMODYNAMICS |
 |
ROCKET THERMODYNAMICS |
A thorough understanding of thermodynamics is not a necessary requirement for the study of rocketry. As long as the temperature, molecular weight, of specific heat ratio of the exhaust products is known, the rocket equations can be solved. It is, however, often useful to be able to derive these quantities for one's self. In this article we provide an overview of the chemical thermodynamics applicable to rocket propulsion. It is assumed the reader has at least an elementary understanding of thermodynamics, as it is not our intent to fully explain the fundamentals.
Because of the complexity and tedious nature of the calculations, an easier method has been provided to readers of this web site. A series of propellant combustion charts are available from which one can obtain optimum mixture ratio, adiabatic flame temperature, gas molecular weight, and specific heat ratio for some common rocket propellants. These charts were developed using the methods described in this article.
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure. The unit of measurement for enthalpy in the International System of Units (SI) is the joule, but other historical, conventional units are still in use, such as the BTU and the calorie.
The enthalpy is the preferred expression of system energy changes in many chemical, biological, and physical measurements, because it simplifies certain descriptions of energy transfer. This is because a change in enthalpy takes account of energy transferred to the environment through the expansion of the system under study.
The total enthalpy, H, of a system cannot be measured directly. Thus, change in enthalpy, ΔH, is a more useful quantity than its absolute value. The change ΔH is positive in endothermic reactions, and negative in heat-releasing exothermic processes. ΔH of a system is equal to the sum of non-mechanical work done on it and the heat supplied to it.
For quasistatic processes under constant pressure, ΔH is equal to the change in the internal energy of the system, plus the work that the system has done on its surroundings. This means that the change in enthalpy under such conditions is the heat absorbed (or released) by a chemical reaction. Enthalpies for chemical substances at constant pressure assume standard state: most commonly 1 bar (0.1 MPa) pressure. Standard state does not, strictly speaking, specify a temperature, but expressions for enthalpy generally reference the standard enthalpy of formation at 25oC (298.15 K).
While H denotes the total enthalpy of a system, h is specific enthalpy, i.e. the enthalpy per mole or unit mass of a substance. We will simply refer to both as enthalpy, since the context will make it clear which is applicable.
The enthalpy of formation, or heat or formation, hof , is the enthalpy of a substance at the base conditions of 25oC and 0.1 MPa pressure. The enthalpy in any other state, relative to this base, would be found by adding the change of enthalpy between 25oC and 0.1 MPa and the given state to the enthalpy of formation. That is, the enthalpy at any temperature and pressure, hT,P, is
where the term (Δh)298,0.1 MPa --> T,P represents the difference in enthalpy between any given state and the enthalpy at 298.15 K, 0.1 MPa. For convenience we usually drop the subscripts in the examples that follow.
In most cases the substances that comprise the reactants and products in a chemical reaction are not at a temperature of 25oC and a pressure of 0.1 MPa. Therefore the change of enthalpy between 25oC and 0.1 MPa and the given state must be known. In the case of a solid or liquid, this change of enthalpy can usually be found from a table of thermodynamic properties or from specific heat data. In the case of gases, this change of enthalpy can be found as follows:
Assume ideal gas behavior between 25oC, 0.1 MPa, and the given state. In this case, the enthalpy is a function of the temperature only, and can be found by use of an equation, from tabulated values of enthalpy as a function of temperature (which assumes ideal gas behavior), or calculated from specific heat capacity, Cpo, where
NIST Chemistry WebBook provides equations for (ho-ho298) and Cpo for most substances, and tables can be generated using the web site NIST-JANAF Termochemical Tables. (ho-ho298) is Δh, where ho298 refers to 25oC or 298.15 K. The superscript o is used to designate that this is the enthalpy at 0.1 MPa pressure, based on ideal gas behavior, that is, the standard-state enthalpy.
When the deviation from ideal gas behavior is significant, other procedures must be employed, but that won't be necessary in our case.
Consider a given combustion process that takes place adiabatically and with no work or changes in kinetic or potential energy involved. For such a process the temperature of the products is referred to as the adiabatic flame temperature. With the assumptions of no work and no changes in kinetic or potential energy, this is the maximum temperature that can be achieved for the given reactants because any heat transfer from the reacting substances and any incomplete combustion would tend to lower the temperature of the products. For a given fuel and given pressure and temperature of the reactants, the maximum adiabatic temperature that can be achieved is with a stoichiometric mixture.
The following example shows how the adiabatic flame temperature may be found. The dissociation that takes place in the combustion products, which has a significant effect on the adiabatic flame temperature, will be considered in the next section.
Suppose we react a stoichiometric mixture of gaseous methane and gaseous oxygen at an initial temperature of 25oC and 4 MPa pressure. Our reaction equation is
We assume ideal gas behavior for all the constituents. In this case the first law of thermodynamics reduces to
where HR is the enthalpy of the reactants, HP the enthalpy of the products, hof the enthalpy of formation of each constituent, Δhi the change in enthalpy of each constituent in the reactants at the initial temperature, and Δhe the change in enthalpy of each constituent in the products at the adiabatic flame temperature.
Below is a table giving enthalpy of formation and ideal gas enthalpy for several of the products that will result from a methane/oxygen reaction. Note that in the simplified reaction considered above, we have only CO2 and H2O on the right side of the equation. The additional substances will appear when we consider dissociation in the next section.
| Temperature K |
Methane CH4 (3/31/61) |
Carbon Dioxide CO2 (9/30/65) |
Carbon Monoxide CO (9/30/65) |
Oxygen, Diatomic O2 (9/30/65) |
Water (gas) H2O (3/31/61) |
Hydrogen, Diatomic H2 (3/31/61) |
Hydroxyl OH (3/31/66) |
| (hfo)298 = -74.873 M = 16.043 |
(hfo)298 = -393.522 M = 44.010 |
(hfo)298 = -110.529 M = 28.010 |
(hfo)298 = 0 M = 31.999 |
(hfo)298 = -241.827 M = 18.015 |
(hfo)298 = 0 M = 2.0159 |
(hfo)298 = 39.463 M = 17.007 |
|
| (ho-ho298) kJ/mol |
(ho-ho298) kJ/mol |
(ho-ho298) kJ/mol |
(ho-ho298) kJ/mol |
(ho-ho298) kJ/mol |
(ho-ho298) kJ/mol |
(ho-ho298) kJ/mol |
|
| 0 | -10.024 | -9.364 | -8.669 | -8.682 | -9.904 | -8.468 | -9.171 |
| 100 | -6.698 | -6.456 | -5.770 | -5.778 | -6.615 | -5.293 | -6.138 |
| 200 | -3.368 | -3.414 | -2.858 | -2.866 | -3.280 | -2.770 | -2.975 |
| 298 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 300 | 0.066 | 0.067 | 0.054 | 0.054 | 0.063 | 0.054 | 0.054 |
| 400 | 3.861 | 4.008 | 2.975 | 3.029 | 3.452 | 2.958 | 3.033 |
| 500 | 8.200 | 8.314 | 5.929 | 6.088 | 6.920 | 5.883 | 5.991 |
| 600 | 13.130 | 12.916 | 8.941 | 9.247 | 10.498 | 8.812 | 8.941 |
| 700 | 18.635 | 17.761 | 12.021 | 12.502 | 14.184 | 11.749 | 11.903 |
| 800 | 24.675 | 22.815 | 15.175 | 15.841 | 17.991 | 14.703 | 14.878 |
| 900 | 31.205 | 28.041 | 18.397 | 19.246 | 21.924 | 17.682 | 17.887 |
| 1000 | 38.179 | 33.405 | 21.686 | 22.707 | 25.978 | 20.686 | 20.933 |
| 1100 | 45.549 | 38.894 | 25.033 | 26.217 | 30.167 | 23.723 | 24.025 |
| 1200 | 53.270 | 44.484 | 28.426 | 29.765 | 34.476 | 26.794 | 27.158 |
| 1300 | 61.302 | 50.158 | 31.865 | 33.351 | 38.903 | 29.907 | 30.342 |
| 1400 | 69.608 | 55.907 | 35.338 | 36.966 | 43.447 | 33.062 | 33.568 |
| 1500 | 78.153 | 61.714 | 38.848 | 40.610 | 48.095 | 36.267 | 36.840 |
| 1600 | 86.910 | 67.580 | 42.384 | 44.279 | 52.844 | 39.522 | 40.150 |
| 1700 | 95.853 | 73.492 | 45.940 | 47.970 | 57.685 | 42.815 | 43.501 |
| 1800 | 104.960 | 79.442 | 49.522 | 51.689 | 62.609 | 46.150 | 46.890 |
| 1900 | 114.212 | 85.429 | 53.124 | 55.434 | 67.613 | 49.522 | 50.308 |
| 2000 | 123.592 | 91.450 | 56.739 | 59.199 | 72.689 | 52.932 | 53.760 |
| 2100 | 133.087 | 97.500 | 60.375 | 62.986 | 77.831 | 56.379 | 57.241 |
| 2200 | 142.684 | 103.575 | 64.019 | 66.802 | 83.036 | 59.860 | 60.752 |
| 2300 | 152.371 | 109.671 | 67.676 | 70.634 | 88.295 | 63.371 | 64.283 |
| 2400 | 162.141 | 115.788 | 71.346 | 74.492 | 93.604 | 66.915 | 67.839 |
| 2500 | 171.984 | 121.926 | 75.023 | 78.375 | 98.964 | 70.492 | 71.417 |
| 2600 | 181.893 | 128.085 | 78.714 | 82.274 | 104.370 | 74.090 | 75.015 |
| 2700 | 191.862 | 134.256 | 82.408 | 86.199 | 109.813 | 77.718 | 78.634 |
| 2800 | 201.885 | 140.444 | 86.115 | 90.144 | 115.294 | 81.370 | 82.266 |
| 2900 | 211.958 | 146.645 | 89.826 | 94.111 | 120.813 | 85.044 | 85.918 |
| 3000 | 222.076 | 152.862 | 93.542 | 98.098 | 126.361 | 88.743 | 89.584 |
| 3200 | 242.431 | 165.331 | 100.998 | 106.127 | 137.553 | 96.199 | 96.960 |
| 3400 | 262.925 | 177.849 | 108.479 | 114.232 | 148.854 | 103.738 | 104.387 |
| 3600 | 283.536 | 190.405 | 115.976 | 122.399 | 160.247 | 111.361 | 111.859 |
| 3800 | 304.248 | 202.999 | 123.495 | 130.629 | 171.724 | 119.064 | 119.378 |
| 4000 | 325.045 | 215.635 | 131.026 | 138.913 | 183.280 | 126.846 | 126.934 |
| 4200 | 345.918 | 228.304 | 138.578 | 147.248 | 194.903 | 134.700 | 134.528 |
| 4400 | 366.855 | 241.003 | 146.147 | 155.628 | 206.585 | 142.624 | 142.156 |
| 4600 | 387.849 | 253.734 | 153.724 | 164.046 | 218.325 | 150.620 | 149.816 |
| 4800 | 408.893 | 266.500 | 161.322 | 172.502 | 230.120 | 158.682 | 157.502 |
| 5000 | 429.982 | 279.295 | 168.929 | 180.987 | 241.957 | 166.808 | 165.222 |
| 5200 | 451.110 | 292.123 | 176.548 | 189.502 | 253.839 | 174.996 | 172.967 |
| 5400 | 472.274 | 304.984 | 184.184 | 198.037 | 265.768 | 183.247 | 180.736 |
| 5600 | 493.469 | 317.884 | 191.832 | 206.593 | 277.738 | 191.556 | 188.531 |
| 5800 | 514.692 | 330.821 | 199.489 | 215.166 | 289.746 | 199.924 | 196.351 |
| 6000 | 535.942 | 343.791 | 207.162 | 223.756 | 301.796 | 208.346 | 204.192 |
| The thermochemical data are calculated from the JANAF Thermochemical Tables, Thermal Research Laboratory, The Dow Chemical Company, Midland, Michigan. The date each table was issued is indicated. |
|||||||
Knowing the temperature of the reactants, we can easily calculate HR. Since the reactant temperature in our example is equal to the base temperature of 25oC, Δhi = 0 and we need only to sum the enthalpies of formation of the reactants. From the table above we see that for diatomic oxygen (hfo)298 = 0, which is typical for diatomic gases, and for methane we find (hfo)298 = -74.873 kJ/mol. Therefore, we have
For the products, we have
By trial-and-error solution, a temperature of the products is found that satisfies this equation. Suppose we've already performed several trials and have begun to hone in on a solution. For the next trial, assume TP = 5,400 K.
Since HP > HR, our assumed temperature is too high. Let's now try TP = 5,200 K.
We can now interpolate to find the adiabatic flame temperature.
Cryogenic Reactants
From the preceding example it can be seen that the initial temperature of the reactants has an effect on the final temperature of the products. For storable propellants, temperature variations within the normal liquid range have only a small effect on engine performance. For hypergols, a 10oC change in reactant temperature results in about 0.1% change in specific impulse. We can, therefore, safely assume that the initial temperature of storable propellants is the standard enthalpy of formation temperature of 25oC. This assumption has the added benefit of simplifying the calculations as we need only look up the enthalpies of formations for the reactants without making any further adjustments for temperature.
Cryogenic propellants are another story, as their extremely low temperatures have a significant effect on performance. We must further take into consideration that the readily available thermochemical data for these substances is gaseous state rather than liquid. An adjustment must be made to account for the change of state.
Let's consider the example of cryogenic oxygen, which has a boiling point of -183oC (90 K). We start with gaseous oxygen at 298 K, which has an enthalpy of formation of 0 kJ/mol. We then add the change in enthalpy required to lower the temperature from 298 K to 90 K. From the enthalpy tables we estimate that this adjustment is -6.06 kJ/mol. Next we deduct the enthalpy of vaporization to account for the change of state from gas to liquid. The enthalpy of vaporization of oxygen is 6.82 kJ/mol. Therefore, the enthalpy of liquid oxygen reactant is
We now turn our attention to chemical equilibrium. Here we will consider a chemical reaction involving only one phase, which is referred to as a homogeneous chemical reaction. In our case we will be considering a gaseous phase, but the basic considerations apply to any phase.
Consider a vessel that contains four compounds, A, B, C, and D, which are in chemical equilibrium at a given pressure and temperature. Let the number of moles of each component be designated nA, nB, nC, and nD. Further, let the chemical reaction that takes place between these four constituents be
where the v's are the stoichiometric coefficients. It should be emphasized that there is a very definite relation between the v's (the stoichiometric coefficients) whereas the n's (the number of moles present) for any constituent can be varied simply by varying the amount of that component in the reaction vessel.
To determine the equilibrium composition for a chemical reaction, we must be able to determine the activity of the various constituents in the mixture. We do so by applying the concept of the equilibrium constant, K. Assuming the ideal gas model, which will be appropriate for our examples and applications, the chemical equilibrium equation can be written as
where y is the equilibrium mole fraction of the A, B, C and D constituents, v is the stoichiometric coefficient for each constituent, and the term P/Po represents the ratio of pressure at which the reaction occurs to the standard state pressure.
The equation is written in this form because it demonstrates quite clearly the influence of various factors on the equilibrium composition (the y's). That is, we know that temperature and pressure both influence the equilibrium composition. It can be seen that the influence of temperature enters through the value of K (which is a function of temperature only) and the influence of pressure though the term (P/Po)VC+VD-VA-VB.
It should be noted that the equilibrium of combustion gases is very sensitive to temperature. Products existing at a high combustion temperature are very different from those existing at a lower combustion temperature. At high temperatures, dissociation of the products occurs, as the thermal energy causes the break up of molecules into simpler and monatomic constituents. Dissociation reactions are reversible (as indicated by the double arrow in the equations), so as the gases cool, recombination can occur.
Let's now revisit our previous example and employ a procedure for determing the equilibrium composition of the combustion products.
It is convenient to view the over-all process as though it occurred in two separate steps, a combustion process followed by a heating and dissociation of the combustion products. The combustion reaction is
There are several dissociation reactions that will come into play, but we'll consider the two most dominant in this particular example.
That is, the energy released by the combustion of CH4 and O2 heats the CO2 and H2O to high temperature, resulting in the dissociation of part of the CO2 to CO and O2, and part of the H2O to H2 and OH. Thus, the overall reaction can be written
where the unknown coefficients a, b, c, d, e, and f must be found by solution of the equilibrium equations associated with the dissociation reactions.
From the combustion reaction we find that the initial compositions for the dissociation reactions are 1 mole CO2 and 2 moles H2O. Therefore, letting z' be the number of moles of CO2 dissociated, and z" be the number of moles of H2O dissociated, we find
CO2 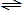 CO + 1/2 O2
Initial: 1 0 0
Change: -z' +z' +z'/2
_____________________________________________
At equilibrium: (1-z') z' z'/2
H2O
CO + 1/2 O2
Initial: 1 0 0
Change: -z' +z' +z'/2
_____________________________________________
At equilibrium: (1-z') z' z'/2
H2O 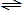 1/2 H2 + OH
Initial: 2 0 0
Change: -z" +z"/2 +z"
_____________________________________________
At equilibrium: (2-z") z"/2 z"
1/2 H2 + OH
Initial: 2 0 0
Change: -z" +z"/2 +z"
_____________________________________________
At equilibrium: (2-z") z"/2 z"
Therefore the overall reaction is
And the total number of moles at equilibrium for each reaction is
The equilibrium mole fractions are
Substituting these quantities along with P = 4 MPa, we have the equilibrium equations.
Values of the equilibrium constants are found in the following table:
| Logarithms to the Base e of the Equilibrium Constant K | ||
| Temperature K |
CO2 = CO + 1/2 O2 | H2O = 1/2 H2 + OH |
| 298 | -103.762 | -106.208 |
| 500 | -57.616 | -60.281 |
| 1000 | -23.529 | -26.034 |
| 1200 | -17.871 | -20.283 |
| 1400 | -13.842 | -16.099 |
| 1600 | -10.830 | -13.066 |
| 1800 | -8.497 | -10.657 |
| 2000 | -6.635 | -8.728 |
| 2200 | -5.120 | -7.148 |
| 2400 | -3.860 | -5.832 |
| 2600 | -2.801 | -4.719 |
| 2800 | -1.894 | -3.763 |
| 3000 | -1.111 | -2.937 |
| 3200 | -0.429 | -2.212 |
| 3400 | 0.169 | -1.576 |
| 3600 | 0.701 | -1.009 |
| 3800 | 1.176 | -0.501 |
| 4000 | 1.599 | -0.044 |
| 4500 | 2.490 | 0.920 |
| 5000 | 3.197 | 1.689 |
| 5500 | 3.771 | 2.318 |
| 6000 | 4.245 | 2.843 |
By trial-and-error solution, a temperature of the products must be found that satisfies the equations. The steps are
Let's assume TP = 3,600 K, we have
Assume TP = 3,800 K and repeat.
Calculate adiabatic flame temperature by interpolation.
Knowing the temperature, we can now interpolate to find the corresponding values of ln(K) and calculate the equilibrium mixture.
For a more accurate solution, there are additional dissociation reactions that have to be considered, such as
It is evident from this rather simple example that accounting for all the possible dissociations requires the solving of a great many simultaneous equations. Consider adding another element to the mixture, such as nitrogen, and we've complicated the problem even further. Providing accurate solutions quickly and easily can only be performed with the aid of a computer program. One such program is the freeware STANJAN, written by former Stanford University Professor William C. Reynolds, © 1984.
Below is the STANJAN solution to the same methane/oxygen example that we've been examining. Note that after all the dissociations have been accounted for, the final adiabatic flame temperature has been lowered to 3,553.87 K.
Independent relative element
system atom population potential
C 1.00000000E+00 -16.8393
O 4.00000000E+00 -14.9514
H 4.00000000E+00 -10.3070
Composition at T = 3553.87 K P = 3.948E+01 atmospheres
species mol fraction mass fraction mol fraction
in the gas in mixture in mixture
gas phase
CO .14455E+00 .17980E+00 .14455E+00
CO2 .13679E+00 .26734E+00 .13679E+00
H .25470E-01 .11400E-02 .25470E-01
HO .93941E-01 .70948E-01 .93941E-01
H2 .58263E-01 .52158E-02 .58263E-01
H2O .44472E+00 .35579E+00 .44472E+00
O .23960E-01 .17023E-01 .23960E-01
O2 .72308E-01 .10275E+00 .72308E-01
Mixture properties: gas molal mass = 22.519 kg/kmol
T = 3553.866 K P = 4.0000E+06 Pa V = 3.2803E-01 m**3/kg
U =-2.2476E+06 J/kg H =-9.3544E+05 J/kg S = 1.1981E+04 J/kg-K
Made 3 (T,P) iterations; 0 condensate iterations; 31 gas passes.
|
The average molecular weight, M, of a mixture is simply
where n is the total number of moles in the mixture, and m is the total mass of the mixture. The total moles and total mass are found by
where ni is the number of moles of each constituent, Mi is the molecular weight of each constituent.
Referring back to the last iteration of our combustion equation, we have
Calculating the molecular weight of the mixture, we have
Alternatively, if the mole fraction, yi, of each constituent is given, where yi = ni / n, the average molecular weight is found by
Referring to the STANJAN run above, we can see that both the mole fraction and the gas molecular weight is given, where M = 22.519 kg/kmol. This is lower than our derived unit because our example failed to take into consideration all the applicable dissociations.
The constant-pressure specific heat, or heat capacity, Cp, and the constant-volume specific heat, Cv, are useful functions for thermodynamic calculations, particularly for gases, and are defined by the following relationships
where h is specific enthalpy, u is specific internal energy, and T is temperature.
A very important relationship between the constant-pressure and constant-volume specific heats of an ideal gas is found in the equation
where Cp and Cv are on a mole basis, and R' is the universal gas constant (8.31446 J/mol-K). This tells us that the difference between the constant-pressure and constant-volume specific heats of an ideal gas is always constant, though both are a function of temperature.
Many thermodynamic solutions use the ratio of the specific heats, k or ![]() , defined as
, defined as
Since it is common for thermodynamic references to provide the value of Cp only, from which Cv is calculated, we can combine equations and write
Values of constant-pressure specific heat are typically found by use of an equation, or from tabulated values of Cp as a function of temperature. The Shomate equation is commonly used, which is shown below along with the Shomate coefficients of the products found in our combustion example.
| Gas Phase Heat Capacity (Shomate Equation) | ||||||
| Cpo = A + Bt + Ct2 + Dt3 + E/t2 where Cp = heat capacity (J/mol-K), t = Temperature (K) / 1000 |
||||||
| Formula | Temp. Range | A | B | C | D | E |
| CO2 | 1200-6000 | 58.16639 | 2.720074 | -0.492289 | 0.038844 | -6.447293 |
| CO | 1300-6000 | 35.15070 | 1.300095 | -0.205921 | 0.013550 | -3.282780 |
| H2O | 1700-6000 | 41.96426 | 8.62253 | -1.499780 | 0.098119 | -11.15764 |
| H2 | 2500-6000 | 43.413560 | -4.293079 | 1.272428 | -0.096876 | -20.533862 |
| H | 298-6000 | 20.78603 | 4.850638x10-10 | -1.582916x10-10 | 1.525102x10-11 | 3.196347x10-11 |
| O2 | 2000-6000 | 20.91111 | 10.72071 | -2.020498 | 0.146449 | 9.245722 |
| O | 2500-6000 | 22.18977 | -1.318044 | 0.378308 | -0.026069 | 0 |
| OH | 1300-6000 | 28.74701 | 4.714489 | -0.814725 | 0.054748 | -2.747829 |
| Shomate coefficients obtained from NIST Chemistry WebBook, except monatomic oxygen (O) derived by R.A. Braeunig from data found here. |
||||||
Calculating the specific heat ratio of a single substance is straightforward. Say we have carbon monoxide at 3,000 K, the specific heat ratio is
When we have a mixture of gases, we must determine the specific heat of the mixture, where
Let's calculate the specific heat ratio for the mixture of gases found in the STANJAN solution to our methane/oxygen problem, noting that the temperature is 3,553.87 K.
Although some computer programs calculate the value of specific heat ratio, STANJAN does not. If STANJAN is used, the user will have to calculate k separately, though this can easily be done through the use of spreadsheet software.
Everything so far has assumed the combustion products consist of a homogenous mixture of gases. When dealing with liquid rocket propellants, this is a fair assumption, as any condensed species (either liquids or solids) will appear in trace quantities only. However, when dealing with solid propellants, a significant portion of the exhaust products will be in a condensed phase, which is evident as visible smoke in the exhaust plume. When the products exist in more than one phase, the mixture is said to be heterogeneous.
Heterogeneity requires that we alter the method by which we calculate the molecular weight and specific heat ratio. The two-phase equations presented below are those derived by Richard Nakka and described in his Experimental Rocketry Web Site.
A key assumption required is that the condensed-phase particles flow at the same velocity as the gas (i.e. no velocity lag), so the modified equations represent an upper limit on performance. The details are too involved to present here, so only the final outcome is presented, which fortunately, is quite simple. As it turns out, the gas-particle mixture behaves like a gas with a modified specific heat ratio, k. All the fundamental equations remain the same and are fully applicable to two-phase flow, with the only modifications being as follows:
where Cpmix is the effective specific heat of the gas and condensed-phase mixture, given by
where ni is the number of moles of gas component i, ns the number of moles of condensed-phase component, and n the total number of gas moles.
kmix is the form to use when calculating combustion chamber pressure and characteristic exhaust velocity.
Where flow velocity and acceleration are high, that is, in the nozzle, we have
where k' is the specific heat ratio for the gas only mixture; Ψ = ws /(1–ws), where ws is the mass fraction of condensed-phase particles in the exhaust; Cs is the specific heat for the solid (or liquid) mixture in the exhaust; and Cpgas is the specific heat for the gas only mixture. As before,
The derivation of this form of modified specific heat ratio assumes a frozen flow condition where no thermal or velocity particle lag is assumed to exist, and is based on the momentum and energy equations for steady isentropic flow.
k2ph is the form to use when calculating exhaust velocity, thrust, thrust coefficient and the other nozzle flow parameters.
Let's now consider an example. Below is a STANJAN run for the combustion of 68% ammonium perchlorate, 18% aluminum, and 14% HTPB at a chamber pressure of 4 MPa.
Independent relative element
system atom population potential
Al 6.67111407E-01 -16.5165
Cl 5.78772661E-01 -20.5197
O 2.31509064E+00 -19.0205
H 3.86805792E+00 -9.2472
C 1.03531152E+00 -12.1297
N 5.78772661E-01 -13.7827
Composition at T = 3280.36 K P = 3.948E+01 atmospheres
species mol fraction mass fraction mol fraction
in the gas in mixture in mixture
gas phase
Al .15875E-03 .15634E-03 .14634E-03
AlCl .82935E-02 .18897E-01 .76450E-02
AlClO .13303E-02 .38081E-02 .12263E-02
AlCl2 .22637E-02 .80879E-02 .20867E-02
AlCl3 .21732E-03 .10576E-02 .20033E-03
AlO .14196E-03 .22269E-03 .13086E-03
AlOH .42786E-03 .68696E-03 .39441E-03
AlO(OH) .29780E-03 .65205E-03 .27452E-03
CO .27553E+00 .28168E+00 .25398E+00
CO2 .81351E-02 .13067E-01 .74990E-02
Cl .82798E-02 .10713E-01 .76324E-02
H .33101E-01 .12178E-02 .30513E-01
HCl .13549E+00 .18031E+00 .12490E+00
HO .30828E-02 .19136E-02 .28418E-02
H2 .36159E+00 .26606E-01 .33332E+00
H2O .82105E-01 .53989E-01 .75685E-01
NO .18270E-03 .20010E-03 .16842E-03
N2 .79197E-01 .80974E-01 .73005E-01
O .16856E-03 .98432E-04 .15538E-03
condensed species
Al2O3(c) .31566E+00 .78192E-01
Mixture properties: gas molal mass = 18.750 kg/kmol
T = 3280.363 K P = 4.0000E+06 Pa V = 2.4894E-01 m**3/kg
U =-2.8456E+06 J/kg H =-1.8499E+06 J/kg S = 9.6821E+03 J/kg-K
Made 3 (T,P) iterations; 11 condensate iterations; 54 gas passes.
|
To perform the calculations, we require the molecular weight and specific heat of each constituent. The following is obtained from the NIST Chemistry WebBook, with Cp valves derived for the chamber temperature of 3280.363 K.
Species M Cp
g/mol J/mol-K
---------------------------------
Al 26.982 20.7692
AlCl 62.435 39.0550
AlClO 78.434 62.2791
AlCl2 97.888 58.3019
AlCl3 133.341 83.0688
AlO 42.981 46.9917
AlOH 43.989 60.2511
AlO(OH) 59.988 81.6723
CO 28.010 37.3728
CO2 44.010 62.5638
Cl 35.453 20.9928
H 1.008 20.7860
HCl 36.461 37.5480
HO 17.007 37.1224
H2 2.016 37.6952
H2O 18.015 56.5356
NO 30.006 37.6115
N2 28.013 37.2055
O 15.999 21.0168
Al2O3(c) 101.961 192.4640
Referring to the "mol fraction in mixture" in the STANJAN run, we find
And from "mass fraction in mixture", we find the mass fraction of the condensed species,
Calculating the total mass, we have
Therefore,
Further, we find
Finally, we calculate the specific heat ratios,
When to use kmix versus k2ph
Referring to the Rocket Propulsion page, when condensed species are present, the following equations should be solved using kmix:
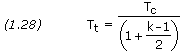
And the following should be solved using k2ph:
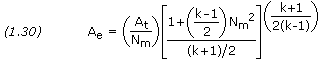
Isentropic Compression & Expansion
Here we present two important equations that relate the pressure, temperature, and volume that a gas occupies during reversible compression or expansion. Such a process occurs during the compression and power strokes for an internal combustion engine. The same equations describe the conditions across the compressor and turbine of a gas turbine, or across the nozzle of a rocket engine. The resulting compression and expansion are reversible processes in which the entropy of the system remains constant. Such a process is called an isentropic process.
For isentropic compression and expansion, the effect of pressure on temperature is described by
and the effect of volume on pressure is
where T1, p1 and v1 are the initial temperature, pressure and volume; T2, p2 and v2 are the final temperature, pressure and volume; and k is the specific heat ratio.
We can also combine equations to find the effect of volume on temperature
Since the expansion of exhaust gases through a rocket nozzle is an isentropic process, the above equations are applicable. Say, for instance, we want to know the temperature at the nozzle exit for a rocket engine having a chamber temperature of 3,250 K, a chamber pressure of 6 MPa, a nozzle exit pressure of 0.07 MPa, and k is known to be 1.23. We have
Should you decide to give STANJAN a try, I have some helpful tips learned through my experience using it. This is not instructions on how to use the software.
First, STANJAN comes with a file named TURBINE.DOC that is an example of a STANJAN run for a gas turbine problem. It outlines the steps for finding adiabatic flame temperature and for finding the results of isentropic expansion of the exhaust. Learn to follow this example and you should be able to perform all the calculations described in this article.
Second, STANJAN comes with a limited number of species data files, so you're certainly going to have to create some of your own if you plan to get serious about using the software. Included with STANJAN is a file named SPECIES.DOC that explains how the data files are formatted. Below is an excerpt showing a sample data entry for carbon dioxide.
Species name (8 chars) Atomic composition: Left justified in Col 1 | number right justified in | Date of data the field followed by | 8 chars starting Col 10 atom symbols (2 chars) | | left justified in the | | Cols 19-28 field (upper or lower | | mol. wt, g/mol case, but you must use | | | the same characters for a | | | cols 29-38 given atom throughout | | | enth. form the data file). Must be | | | at 298.15K, positioned as below; | | | kcal/mol 6(I3,A2) starting in | | | | col 49. | | | | Cols 39-48 . | | | | 0.0 for gas; . | | | | density, g/cc . | | | | for liquid or . | | | | solid ............................... | | | | | | | | | | | | | | | | | .. | .. | .. | .. | .. | .. CO2 9/30/65 44.00995 -94.054 0.000 1C 2O 0 0 0 0 47.769 51.072 51.127 53.830 56.122 58.126 59.910 61.522 62.992 64.344 65.594 66.756 67.841 68.859 69.817 70.722 71.578 72.391 73.165 73.903 74.608 75.284 75.931 76.554 77.153 77.730 78.286 78.824 79.344 79.848 80.336 80.810 81.270 81.717 82.151 82.574 82.986 83.388 83.780 84.162 84.536 84.901 85.258 85.607 85.949 86.284 86.611 86.933 87.248 87.557 87.860 88.158 88.451 88.738 89.021 89.299 89.572 89.841 90.106 90.367 -0.816 0.000 0.016 0.958 1.987 3.087 4.245 5.453 6.702 7.984 9.296 10.632 11.988 13.362 14.750 16.152 17.565 18.987 20.418 21.857 23.303 24.755 26.212 27.674 29.141 30.613 32.088 33.567 35.049 36.535 38.024 39.515 41.010 42.507 44.006 45.508 47.012 48.518 50.027 51.538 53.051 54.566 56.082 57.601 59.122 60.644 62.169 63.695 65.223 66.753 68.285 69.819 71.355 72.893 74.433 75.976 77.521 79.068 80.617 82.168 |
STANJAN already includes the gaseous species you'll commonly encounter in most combustion reactions, that is, those containing C, H, N and O. This will suffice for calculations involving most liquid propellants. However, if you plan to perform calculations involving solid propellants, new elements will be introduced into the exhaust, such as Al and Cl. This will necessitate the creation of an entirely new group of species data files.
Data files can be easily edited and/or created in a text editor such as Notepad or WordPad. The values that populate the data files can be found and/or calculated using NIST Chemistry WebBook or, even easier, using NIST-JANAF Thermochemical Tables. Be advised, however, that the STANJAN data files use units of calories instead of Joules. The NIST Chemistry WebBook allows the selection of either calorie-based or Joule-based data. The NIST-JANAF Thermochemical Tables site, although presenting the data in a more convenient format, must be converted from Joules to calories, where 1 calorie = 4.184 Joules.
You will most definitely need to create you own data files for the reactants, as STANJAN doesn't include any of the common rocket propellants. However, there's a short cut that greatly simplifies this task. If you always assume that your reactants are at an initial temperature of 298.15 K, i.e. the base temperature, there is no need to populate the data files with all the S and H-H(298.15) data, as this is used only when the temperature varies from 298.15 K. You need to complete the data on the first line only, making your reactant data files look like the following example for methyl hydrazine:
CH6N2 1951 46.07170 12.940 0.866 1C 6H 2N 0 0 0
.000 .000 .000 .000 .000 .000 .000 .000 .000 .000
.000 .000 .000 .000 .000 .000 .000 .000 .000 .000
.000 .000 .000 .000 .000 .000 .000 .000 .000 .000
.000 .000 .000 .000 .000 .000 .000 .000 .000 .000
.000 .000 .000 .000 .000 .000 .000 .000 .000 .000
.000 .000 .000 .000 .000 .000 .000 .000 .000 .000
.000 .000 .000 .000 .000 .000 .000 .000 .000 .000
.000 .000 .000 .000 .000 .000 .000 .000 .000 .000
.000 .000 .000 .000 .000 .000 .000 .000 .000 .000
.000 .000 .000 .000 .000 .000 .000 .000 .000 .000
.000 .000 .000 .000 .000 .000 .000 .000 .000 .000
.000 .000 .000 .000 .000 .000 .000 .000 .000 .000
|
Of course, assuming a temperature of 298.15 K appears, at first, to be a problem when using cryogenic propellants, but there's a way around this. Besides, STANJAN allows a minimum starting temperature of 200 K, so we can't set our reactants to cryogenic temperatures even if we wanted to. Furthermore, STANJAN doesn't allow reactants of different temperatures, so it can't handle a scenario like RP-1 and LOX, where the temperatures are vastly different.
The solution is a simple trick; in the field where you would normally enter the enthalpy of formation, replace this with the enthalpy of the cryogenic liquid at its boiling point. I earlier demonstrated this when calculating the enthalpy of LOX to be -12.88 kJ/mol, or -3.078 kcal/mol. Now when entering a temperature of 298.15 K, you'll actually get the enthalpy of the cryogenic liquid.
Finally, I like to create separate data files for the reactants and the products. And for the products I like to create several data files, each one representing a different group of elements. For example, I have data files HO.DAT, CHO.DAT, CHNO.DAT, etc. Thus, if my reaction contains only the elements C, H and O, I'll select the file CHO.DAT, which contains all the constituents normal to such a reaction – C, CO, CO2, H, H2, HO, H2O, O and O2. This reduces the number of keystrokes require versus having to select the applicable constituents from an all-inclusive list of products.
STANJAN will operate on a PC running Windows XP or earlier. It will also work with Windows 7 Professional in XP Mode. Whether or not it will run on other platforms is unknown.
Compiled, edited and written in part by Robert A. Braeunig, 2012.
Bibliography